Why Is Graphite Not the Best Conductor of Electricity
Different materials will respond differently when charged or exposed to the presence of a nearby charged. The movement of electrons and ions in them is permitted by a conductor.
Why Is Graphite Not The Best Conductor Quora
The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver.

. Full PDF Package Download Full PDF Package. If the thermal paste can conduct electricity then it may lead to the short circuitry. The carbon atoms are sp2 hybridized.
Which among iron and mercury is a better conductor of electricity. It has a metallic luster. Each atom in a graphene sheet is connected to its three nearest neighbors by a strong.
We can control them by using insulators which make it hard for them to flow and conductors which make it easy. Both have chemical formula yet they do not have a molecular formula. Metal is a good heat conductor because it lets heat flow through it easily.
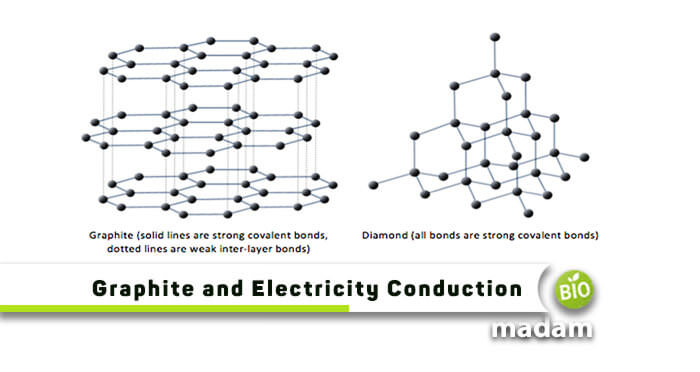
The electron stable state configuration in atoms is best seen in the _____ configuration. People also downloaded these PDFs. Whereas in diamond they have no free mobile electron.
A short summary of this paper. Take a peek at our comparison chart and buyers guide to get all the information you need to make a sound purchasing decision. The best approach to apply the paste is to take a pea-sized amount at the center and allow the heatsink to push the paste on the CPU.
Purcell electricity and magnetism. Diamond and graphite are both large covalent materials made totally of carbon atoms. This is because the electrons of silver metal are available for free movement when compared to the other metals.
Electricity and heat are different forms of energy. Which carbon compound is soft slippery a decent electricity conductor and bonded in a way that forms planes. This technique avoids spreading of paste over the heat spreader it works well with a less viscous paste.
It has reduced melting point. Graphene illustrated in Figure 8 is not only strong and lightweight but it is also an excellent conductor of electricity and heat. Both of a conductors ends are at the same potential.
This fun KS2 science quiz tests Year 3 Year 4 Year 5 and Year 6 students understanding of how. A conductors charge density is zero. All materials are generally placed into two categories - those that are conductors and those that are insulators.
Though this usually involves direct physical contact electrical contact between the two metals may be achieved indirectly through the use of an insulation-coated conductor structural steelwork or any other conductive material. Each conductor as it moves around with the armature experiences a force whose time variation is a replica of the flux densityB. Insulators do not allow for the free flow of electrons across their surface.
Therefore the average conductor force. Name the material which is the best conductor of electricity. The sulfuric acid is not consumed or react with the reactant.
The heat flows the heated atoms dont. Active conductor length and I c conductor current 1. Great conductor of electricity.
The force on the conductors is unidirectional. Full d shell full s shell inert. Diamond petroleum coal graphite.
Silver is not always an ideal choice as a material however because it is expensive and susceptible to tarnishing. Free charges occur only on the surface of the conductor. 14 Full PDFs related to this paper.
Many metals are strong conductors of electricity. When heat travels by conduction through a heat conductor the heat is passed on from one atom to the next. It is not necessary for the connection medium to be exposed to the electrolyte for corrosion to occur.
Top 5 Metal Melting Furnaces For Gold Silver Copper. Graphene ˈ ɡ r æ f iː n is an allotrope of carbon consisting of a single layer of atoms arranged in a two-dimensional honeycomb lattice nanostructure. USA Cast Master GG 5000 SS Best All-Around Choice 2.
Thats why we rounded up a collection of some of the best metal melting furnaces on the market. Silver metal is the best conductor of electricity. F c av Bav l I c where Bav average flux density over a pole.
Copper conducts heat ten times better than iron. Lithium-ion battery chemistry As the name suggests lithium ions Li are involved in the reactions driving the batteryBoth electrodes in a lithium-ion cell are made of materials which can intercalate or absorb lithium ions a bit like the hydride ions in the NiMH batteriesIntercalation is when charged ions of an element can be held inside the structure of. So it has been said that diamonds are bad conductor electricity.
A conductors electrical field is zero allowing electrons to pass inside it. People also downloaded these free PDFs. Conductors are types of materials that allow electrons to flow freely across their surfaces.
The name is derived from graphite and the suffix -ene reflecting the fact that the graphite allotrope of carbon contains numerous double bonds. Copper and silver are the best heat conductors. In a graphite molecule one valence electron of each carbon atom remains free Therefore graphite gained a name of a good conductor of electricity.
Among iron and mercury iron. These properties may prove very useful in a wide range of applications such as vastly improved computer chips and circuits better batteries and solar cells and stronger and lighter structural materials.

Bond Why Is Fullerene 60 An Insulator While Graphite Is A Conductor Chemistry Stack Exchange

Answer The Following Question Why Is Graphite A Good Conductor Of Electricity But Not Diamond
Is Graphite A Good Conductor Of Electricity Biomadam

Answer The Following Question Why Is Graphite A Good Conductor Of Electricity But Not Diamond
No comments for "Why Is Graphite Not the Best Conductor of Electricity"
Post a Comment